Abstract
Introduction: Tirabrutinib (TIRA; GS/ONO-4059) is a Bruton's tyrosine kinase (BTK) inhibitor. Idelalisib (IDELA), a first-in-class phosphatidylinositol-3-kinase delta (PI3Kδ) inhibitor, is approved for the treatment of CLL. Entospletinib (ENTO) is a selective inhibitor of spleen tyrosine kinase (SYK). All three have single agent activity in CLL and updated results from TIRA monotherapy and the combinations of TIRA+IDELA and TIRA+ENTO from this ongoing phase 1b study (NCT02457598) are reported here.
Methods: Patients with previously treated CLL and no prior exposure to targeted inhibitors were eligible for enrollment. For the TIRA+IDELA combination, patients were treated with dose levels in a 3+3 approach combining either idelalisib 50 mg BID or 100mg QD and TIRA ranging from 20mg to 80mg QD. For the TIRA+ENTO combination, patients were treated with entospletinib at either 200mg or 400mg QD and tirabrutinib ranging from 40mg to 80mg QD in dose levels with a 3+3 approach. TIRA monotherapy was with 80mg QD.
Results: As of March 5, 2018, total of 50 CLL patients have been enrolled, 26 patients in the TIRA monotherapy group, 14 in the TIRA + IDELA group, and 10 patients in the TIRA + ENTO group. The median number of prior therapies is 1 (range 1-6). No MTD was identified for either combination at the doses evaluated and activity was high at all dose levels.
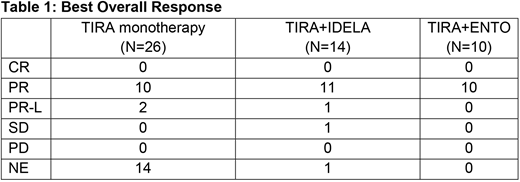
For the 25 patients who have received at least one dose of TIRA monotherapy, the median duration of treatment is 28 weeks (range 0.3-54.1), and 22 patients are still on treatment. All 12 patients evaluable for response per IWCLL2008 criteria achieved a partial response on study with best overall response shown in table 1. Treatment-emergent adverse events (TEAE) were reported in 92% patients, with 28% having a ≥grade 3 TEAE. The most common TEAEs (any grade/≥grade 3) were diarrhoea (24%/0), constipation (20%/0), neutropenia (16%/12%), contusion (12%/0), asthenia (12%/0), ecchymosis (12%/0), and nausea (12%/0). AEs led to treatment interruption in 3 patients and discontinuation in 2 patients and there were no deaths in this group on study.
For the 14 patients in the TIRA+IDELA group, the median duration of treatment is 100 weeks (range 36-134.9), and 11 patients are still on treatment. 12/13 evaluable patients achieved a response on study with best overall response shown in table 1. TEAEs were reported in all patients with 64% of patients having a ≥grade 3 TEAE. The most common TEAEs (any grade/≥grade 3) were diarrhoea (43%/7%), neutropenia (36%/36%), bronchitis (36%/0), rash (36%/0), back pain (29%/0), dyspepsia (29%/0), nausea (29%/0), cough (29%/0), constipation (29%/0), arthralgia (29%/0), pruritus (29%/0). AEs led to interruption or discontinuation of all study treatment in 5 and 1 patients, respectively. One patient died due to sudden respiratory difficulty not believed to be related to study treatment.
For the 10 patients in the TIRA+ENTO group, the median duration of treatment is 82 weeks (range 57.1-93.9) and all patients are still on treatment. All patients achieved a partial response on study with best overall response shown in table 1. TEAEs were reported in all patients with 60% having a ≥grade 3 TEAE. The most common TEAEs (any grade/≥grade 3) were contusion (50%/0), fatigue (50%/0), diarrhoea (40%/0), upper respiratory tract infection (40%/10%), rhinitis (30%/0), alanine aminotransferase increased (30%/10%), cough (30%/0), and dyspepsia (30%/0%). AEs led to interruption or discontinuation of all study treatment in 4 and 0 patients respectively.
Conclusion: Tirabrutinib in combination with idelalisib or entospletinib at the doses evaluated was tolerable with no significant potentiation of already characterized side effects from the single agents such as bleeding, diarrhea or cytopenias. Both combinations showed a high level of activity in CLL and are currently being evaluated in Phase 2 studies.
Danilov:Bayer Oncology: Consultancy, Research Funding; Aptose Biosciences: Research Funding; TG Therapeutics: Consultancy; Verastem: Consultancy, Research Funding; Astra Zeneca: Consultancy; Genentech: Consultancy, Research Funding; Takeda Oncology: Research Funding; Gilead Sciences: Consultancy, Research Funding. Herbaux:Gilead Sciences, Inc.: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Dyer:Gilead Sciences, Inc.: Honoraria, Research Funding. Hillmen:Celgene: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences, Inc.: Honoraria, Research Funding; Pharmacyclics: Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Alexion Pharmaceuticals, Inc: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Rule:Kite: Membership on an entity's Board of Directors or advisory committees; Celltrion: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau. Huang:Gilead Sciences, Inc.: Employment. Mitra:Gilead Sciences, Inc.: Employment. Karlin:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support. Fegan:Napp: Honoraria; Abbvie: Honoraria; Gilead Sciences, Inc.: Honoraria; Roche: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal